Merit Recognizes National Kidney Month & World Kidney Day

National Kidney Month and World Kidney Day are here, and both are very important times we recognize at Merit Medical. All over the globe, you will find the Merit family raising awareness about kidney disease as well as continuing to ensure our dialysis products and education services are available to clinicians and their patients.
Kidney disease is a global issue. Approximately 2 million people worldwide suffer from kidney failure, and the number of patients diagnosed with the disease continues to increase at a rate of 5—7% per year.1 As many people worldwide currently receive treatment with dialysis or a kidney transplant to stay alive, yet this number may only represent 10% of people who actually need treatment to live.2
Through our dedication and the supportive programs, high-quality products, and services we provide, our team acts as an ambassador for better dialysis care. Here’s how.
OUR DEDICATION
Our Dialysis team is made up of expert clinical support specialists, sales representatives, portfolio managers, and more—all dedicated to ensuring our products and services are made available to the clinicians and patients who need them.

OUR PRODUCTS
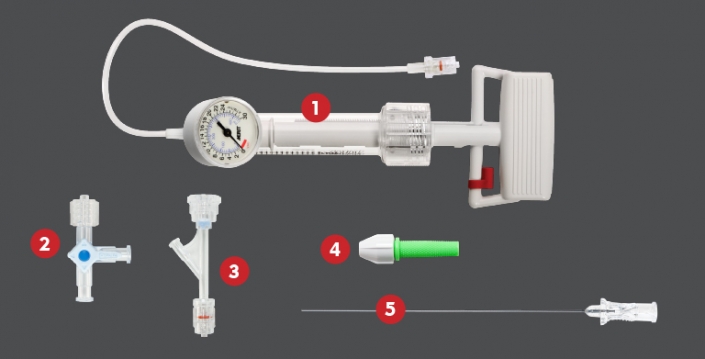
Our diverse portfolio of dialysis access products provides what clinicians need from stick to stitch.
This includes reliable hemodialysis options, such as the HeRO® Graft—the only fully subcutaneous AV access solution clinically proven to maintain long-term access for hemodialysis patients with central venous stenosis.
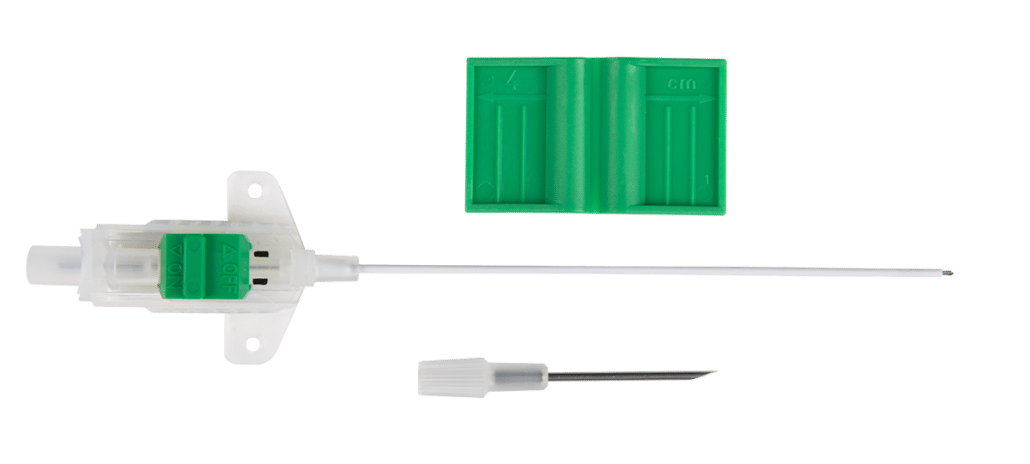
For peritoneal dialysis (PD) care, we offer state-of-the-art Flex-Neck® Peritoneal Dialysis Catheters. With a larger internal diameter over competitive catheters, Flex-Neck catheters allow for higher flow rates than other catheters on the U.S. market.3 Catheter Implantation Kits are also available, designed to streamline the implantation procedure and promote efficiency.
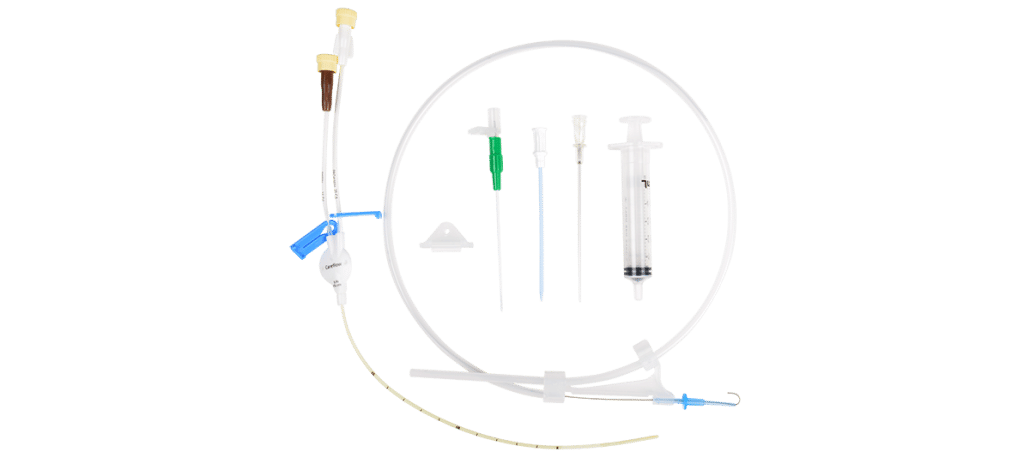
Clinicians can also choose from an assortment of other hemodialysis products, such as the Centros® Hemodialysis Catheters or ProGuide™ Chronic Dialysis Catheters as well as innovative renal accessories, such as the Slip-Not® Suture Retention Device.
Browse through our entire collection of Dialysis Access Solutions.
OUR SERVICES
Clinician Training
At Merit, our goal is to not only provide high-quality dialysis products but also offer the training and support needed for clinicians to give patients optimal dialysis care. In turn, we also offer helpful resources patients can use to familiarize themselves with all dialysis treatment options.
To adapt our training options during the pandemic, we offer a Virtual Percutaneous PD Webinar for physicians and a Virtual Dialysis Access CE Program for nurses, dialysis technicians, and other healthcare professionals.
Visit us here to register for these virtual training opportunities as well as learn about our Think Dialysis Access™ physician education program.
Clinical Case Support
To better aid clinicians in dialysis access cases, we also have a dedicated team of dialysis access clinical specialists who understand the intricacies of hemodialysis and PD and are ready to support. From explaining correct Merit product use to on-site troubleshooting, Merit clinical specialists are available to assist.
Patient Education: Ask4PD

A significant part of improving dialysis care is ensuring patients understand their treatment options. Ask4PD.com is a patient education site powered by Merit that’s dedicated to helping patients understand dialysis care, especially PD. Individuals have access to helpful resources, including patient and healthcare professional testimonials.
MORE RESOURCES
Learn more about kidney health, kidney disease, and treatment with these helpful resources:
- National Institute of Diabetes and Digestive and Kidney Diseases (NIH)
- American Association of Kidney Patients
- American Kidney Fund
- DaVita
- American Renal Associates
- Dialysis Patient Citizens
- Fresenius Kidney Care
- Home Dialysis Central
This National Kidney Month, World Kidney Day, and every day, our team at Merit is dedicated to being your partner in providing better dialysis care.
Explore our complete Dialysis Access portfolio and connect with our Customer Support Center to see how you can incorporate these solutions into your dialysis practice today.
References
- University of California San Francisco. 2018. “The Kidney Project.” https://pharm.ucsf.edu/kidney/need/statistics
- National Kidney Foundation. n.d. “Global Facts: About Kidney Disease.” https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease#:~:text=Over%202%20million%20people%20worldwide,actually%20need%20treatment%20to%20live.
- Katzman HE et al. 2009. “Initial Experience and Outcome of a New Hemodialysis Access Device for Catheter-Dependent Patients. J Vasc Surg Sep;50(3):600-607
New SplashWire™ Delivers Superior Performance in Diagnostic and Interventional Procedures
Merit Medical is proud to announce the launch of the SplashWire™, a hydrophilic steerable guide wire designed to facilitate the placement of devices during diagnostic and interventional procedures. The SplashWire is the result of Merit’s commitment to continued innovation and dedication to making superior products that meet the specific needs of physicians.
A number of key features enable the SplashWire to attenuate common issues associated with guide wire use—such as wire whipping and friction—as well as promote rapid catheter exchanges, all helping to traverse difficult lesions and support successful clinical outcomes in the most challenging procedures.
KEY FEATURES
Resilient Hydrophilic Coating—Provides a constant lubricity that’s superior to the market-leading hydrophilic guide wire,1 enhancing lesion crossability and reducing friction and insertion forces when traversing complex lesions.
Tungsten-Infused Polymer Jacket—Combined with the nitinol core wire, boosts visualization under fluoroscopy for accurate positioning.
Multiple Configurations—For added procedural flexibility, the SplashWire is available in many diameters, lengths, and angled and straight configurations.
Stiff Configuration—Offers more support and pushability.
Nitinol Core—Delivers kink resistance and true 1:1 torque response for smooth navigation in tortuous vessels.1
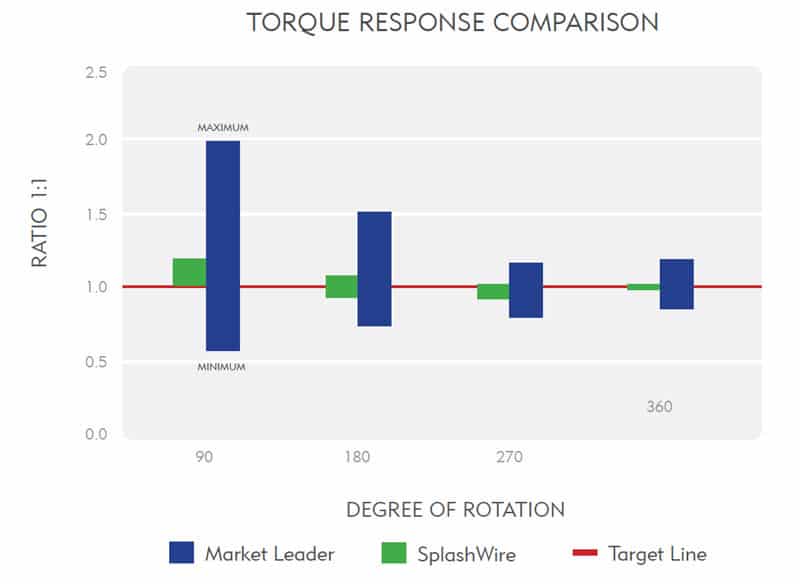
Comparative torque testing1 shows the SplashWire consistently meets the 1:1 torque target, unlike the market leader’s exaggerated torque response. As displayed in the chart, bars closest to the target line indicate true 1:1 torqueability.



The SplashWire is a valued addition to the Merit Angiography portfolio, a compilation of tools developed by applying physician feedback, investing in new technologies, and improving upon legacy products. This dedication to continued innovation and ongoing excellence has resulted in products that deliver superior performance for diagnostic and interventional procedures.
Learn more about the SplashWire Hydrophilic Steerable Guide Wire by exploring its product page or by connecting with our Customer Support Center to see how you can incorporate it and other Merit solutions into your practice today.
References
1. Test data on file.
ClariVein® OC Provides Varicose Vein Relief, Less Pain Than Radiofrequency Ablation, Similar Quality of Life Outcomes
Varicose veins are a common condition worldwide. Currently estimated to affect up to 3 in 10 adults,1 varicose veins are responsible for significantly diminished quality of life with consequent healthcare costs.2
To treat varicose veins, endovenous thermal ablation has been considered the gold standard of care—but this technique is not without patient discomfort. In recent years, new therapies have been developed and research implemented in an effort to improve the treatment experience for patients with varicose veins.
A multi-centre randomized controlled trial2 by researchers at Imperial College London and London North West Hospitals NHS Trust found that compared with radiofrequency ablation—a type of endovenous thermal ablation—treatment with minimally invasive ClariVein OC was less painful and produced similar short-term results as well as quality of life and safety outcomes.
The study,2 published in Phlebology, recruited 170 patients and randomly assigned them to undergo either radiofrequency ablation or treatment with ClariVein OC. Researchers examined pain scores using a Visual Analogue Scale (VAS) and number scale (0 ̶ 10) during treatment. Patients were followed up at one and six months with clinical scores, quality of life scores, and ultrasound assessment of the treated leg.
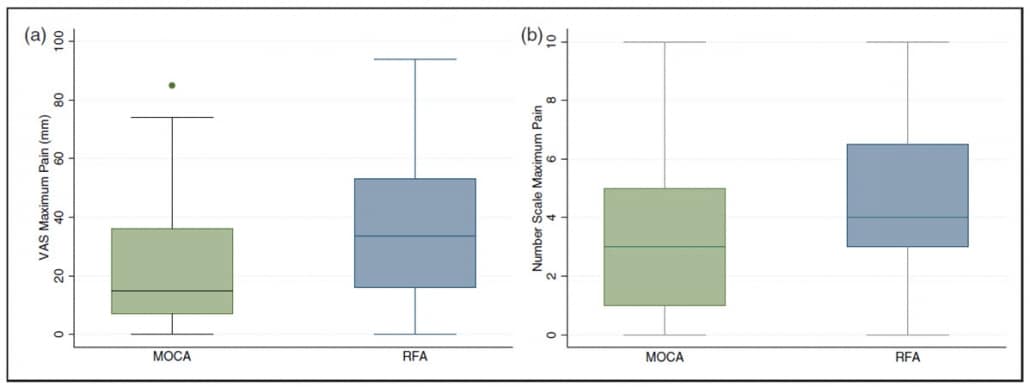
Results2 showed that patients in the ClariVein OC group experienced significantly less pain during the procedure by both VAS (ClariVein OC median 15 mm [IQR 7–36 mm] vs. radiofrequency ablation 34 mm [IQR 16–53 mm], p = 0.003) and number scale (ClariVein OC median 3 [IQR 1–5] vs. radiofrequency ablation 4 [IQR 3–6.5], p = 0.002).
Data2 also showed that average pain scores were significantly less in the ClariVein OC group (VAS median 10 mm [3–25 mm] vs. 19.5 mm [9–38 mm], p = 0.003, Mann-Whitney, and number scale median of 2 [0.5–4] versus 3 [2–5], p = 0.004, Mann Whitney).
Occlusion rates, clinical severity scores, disease specific and generic quality of life scores were similar between groups at one and six months.2 Overall, patients returned to work and normal activities at a median of two days (IQR 2–7 and 1–6, respectively). Adverse events included two deep vein thromboses, one in each group.
The researchers concluded2 that treatment for varicose veins is less painful with ClariVein OC than radiofrequency ablation with similar short-term technical, quality of life, and safety outcomes. Clinical outcomes and time returning to work and normal activities were all similar. These findings support other data comparing patient pain between ClariVein OC and radiofrequency ablation3 as well as published five-year follow-up data examining ClariVein OC’s long-term efficacy.4
Varicose veins can be unsightly and for some patients cause discomfort. The underlying cause of varicose veins is venous reflux disease, which occurs when the valves inside veins become weakened or damaged. When this happens, blood flows backwards and collects inside the vein, causing it to enlarge. Varicose veins are most common in the legs and feet. Left untreated, venous reflux disease can progress to create other more serious circulatory and skin problems.
To treat varicose veins, radiofrequency ablation uses imaging guidance to navigate a small catheter into the diseased vein to deliver heat, causing vessel walls to collapse and close. Although considered effective, radiofrequency ablation requires the use of tumescent anaesthesia which involves multiple needle injections.
In contrast, ClariVein OC combines the power of a specialty infusion catheter with a rotating wire tip designed for the controlled 360-degree dispersion of physician-prescribed medication inside the targeted vessel. Requiring only a pin-sized entry through the skin, treatment with the ClariVein OC takes very little time, uses no heat or tumescent anaesthesia, and creates minimal discomfort to the patient.
Learn more about the clinically proven ClariVein OC and how it can change the lives of your patients. Visit ClariVein.com or contact our Customer Support for more information.
References
- NHS Inform. 13 February 2020. “Varicose Veins.” https://www.nhsinform.scot/illnesses-and-conditions/heart-and-blood-vessels/conditions/varicose-veins
- Lane et al. 2016. “A Multi-Centred Randomised Controlled Trial Comparing Radiofrequency and Mechanical Occlusion Chemically Assisted Ablation of Varicose Veins—Final Results of the Venefit versus ClariVein for Varicose Veins Trial.” Phlebology 32, no. 2 (March): 89–98. PMID: 27221810.
- Vun et al. 2015. “Lower Pain and Faster Treatment with Mechanico-Chemical Endovenous Ablation Using ClariVein®.” Phlebology 30, no 10 (Dec): 688–692. PMID: 25300311.
- Mirandola et al. 2020. “An Italian Experience with Mechanochemical Ablation of the Saphenous Vein Since 2012.” J Vasc Surg Venous Lymphat Disord 8, no 6 (Nov): 999–1005. PMID: 32179039.
Introducing the SafeGuard Focus™, Revolutionary Compression for Pacemakers and ICD Pockets

Merit Medical is proud to announce the launch of the SafeGuard Focus, a revolutionary compression device designed specifically for use in patients with pacemakers and implantable cardioverter defibrillator (ICD) pockets. Available in two unique versions—a contoured adhesive dressing and an adhesive-free flexible strap—the SafeGuard Focus is designed to provide accurate and quick hemostasis that is also comfortable for the patient.
Pacemakers and ICDs are placed in a “pocket” under the skin as a therapeutic option to monitor, detect, and control abnormal heart rhythms. To provide compression care to this patient population, electrophysiologists, nurses, and other healthcare professionals will often utilize pocket compression products on the market that fall short of effective, turn to handmade pressure bandages as an alternative, or rely on antithrombin products that can result in potential side effects1 for the patient.
Listening to physician feedback, Merit created the SafeGuard Focus as a reliable solution to this common clinical challenge, designed to reduce the risk of pocket bleeding2 and the risk of subsequent infection.1,3
Advanced SafeGuard Focus features and benefits include:

More Targeted Pressure
Delivers nearly 2x the pressure versus handmade compression devices.4,5
Flexible Compression
Adjustable pressure allows desired compression while maintaining patient comfort.
Ease of Use
Quick application without adjusting the patient’s position.*
Unobstructed Site Visibility
A clear window facilitates surgical site assessment without device removal.
Durable Attachment
Medical grade adhesive backing (adhesive version)** and a VELCRO® brand strap (adhesive-free version) offer secure attachment.
Patient Comfort
Soft thermoplastic balloon (both versions) and nylon loop (non-adhesive version) enhance patient comfort.
Merit is committed to understanding the clinical challenges healthcare providers face, then innovating and delivering advanced product solutions that address these needs. The SafeGuard Focus Compression Device is one such solution, a revolutionary addition to the Merit Hemostasis portfolio and to electrophysiology labs worldwide.
Learn more about the SafeGuard Focus by exploring its product page or by connecting with our Customer Support Center to see how you can incorporate it and other Merit hemostasis solutions into your electrophysiology practice today.
REFERENCES
- Essebag V et al. 2016. “Clinically Significant Pocket Hematoma Increases Long-Term Risk of Device Infection.” Journal of the American College of Cardiology 67, no. 11 (March): 1300–1308. PMID: 26988951.
- American Heart Association. 2000. “ECC Guidelines Part 5: New Guidelines for First Aid.” Circulation 102 (suppI_1): I-77–I-85.
- Baddour LM et al. 2010. “Update on Cardiovascular Implantable Electronic Device Infections and Their Management—A Scientific Statement from the American Heart Association and on behalf of the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young.” Circulation121: 458–477. PMID: 20048212.
- SafeGuard Focus SP 0522.X Revision 001 Input/Output Specification Matrix (ECN145093).
- Design Verification for SafeGuard Focus REP 20-0118F.00101 Revision 001 Final Report (ECN144963).
* Adhesive version only.
** The adhesive portion of the SafeGuard Focus should not be used over damaged skin.
VELCRO® is a registered trademark of Velcro IP Holdings LLC. Used with permission.
Battling Flu Season and COVID-19, Merit Medical Provides Critical Care Products to ICUs
It is officially flu season in the Northern Hemisphere. In the US alone, the Centers for Disease Control and Prevention estimates that influenza (or flu) has caused between 140,000–810,000 hospitalizations annually since 2010. On top of that, COVID-19 cases are surging once again. To help hospitals battle flu season and the uptick in COVID-19 cases, Merit Medical provides the products they need to care for these vulnerable patients in the intensive care unit (ICU).
CENTRAL VENOUS CATHETERS IN CRITICAL CARE
A central venous catheter (CVC), otherwise known as a central line, is commonly placed in a large vein in the neck, chest, arm, or groin to perform quick medical tests or to administer medication, fluids, or blood products. CVCs are an option for patients in the ICU, as they can remain for longer periods of time than standard intravenous (IV) accesses, providing access for those who may be receiving treatment several times each day.
“Often times CVCs are used with critically ill patients because access to viable peripheral veins is compromised,” explains Stephanie Olsen, RN, Director of Global Marketing at Merit Medical. “CVCs are more stable and durable. Where standard peripheral IVs are required to be changed every 72 hours—and may not remain patent as long as a central line access will—Merit CVCs don’t need to be changed out as often, lasting up to 29 days.”
MERIT MEDICAL PRODUCT PICKS
Secalon-T™
Key Features & Benefits:
- Designed for high-flow rate infusions
- PTFE or FEP material construction with Floswitch®—ideal for rapid short-term insertion into central veins or large peripheral vessels
- Available in a kit with pre-puncture stylus and stabilizing wing
Careflow™ Central Venous Catheters
Key Features & Benefits:
- Designed for catheterization via the Seldinger technique
- Tapered atraumatic tip designed to improve catheter insertion and reduce the risk of venous damage
- Streamlined junction hub enables ease of catheter suture and comfortable positioning on patient’s skin
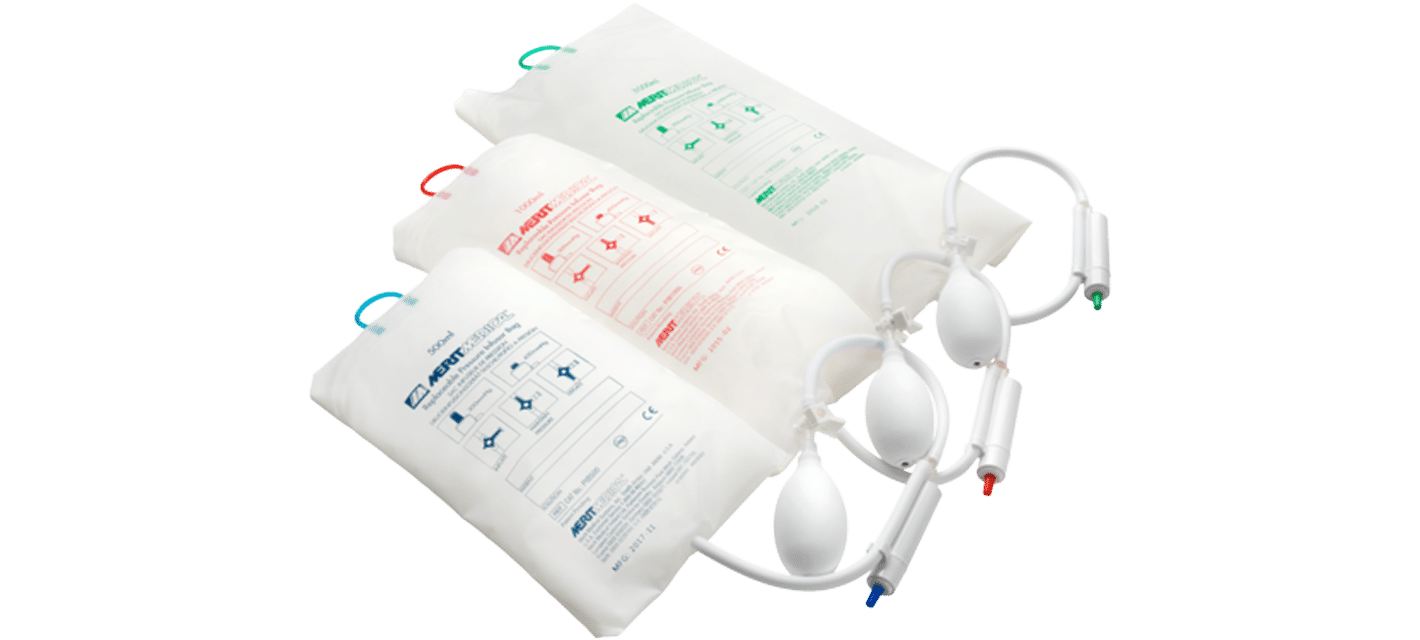
PRESSURE INFUSOR BAGS IN THE ICU
Pressure infusor bags are a valuable solution for injured or critically ill patients who require rapid administration of fluids or blood products. Pressurized bags also prevent the backflow of fluid into the catheters and tubing during treatment or procedures.
“Pressurized bags ensure patients receive the rapid treatment they need while also freeing up the nursing staff’s valuable time, as manually squeezing a bag is not necessary when using a pressure infusor bag,” Olsen says.
MERIT MEDICAL PRODUCT PICK
Pressure Infusor Bag™
Key Features & Benefits:
- Dual-pressure safety valve switches between normal and over-pressure mode with a click of a button
- Pressurization and deflation can be achieved with one hand
- Blunt hook will not puncture the fluid bag and is secure in the event of pressure loss
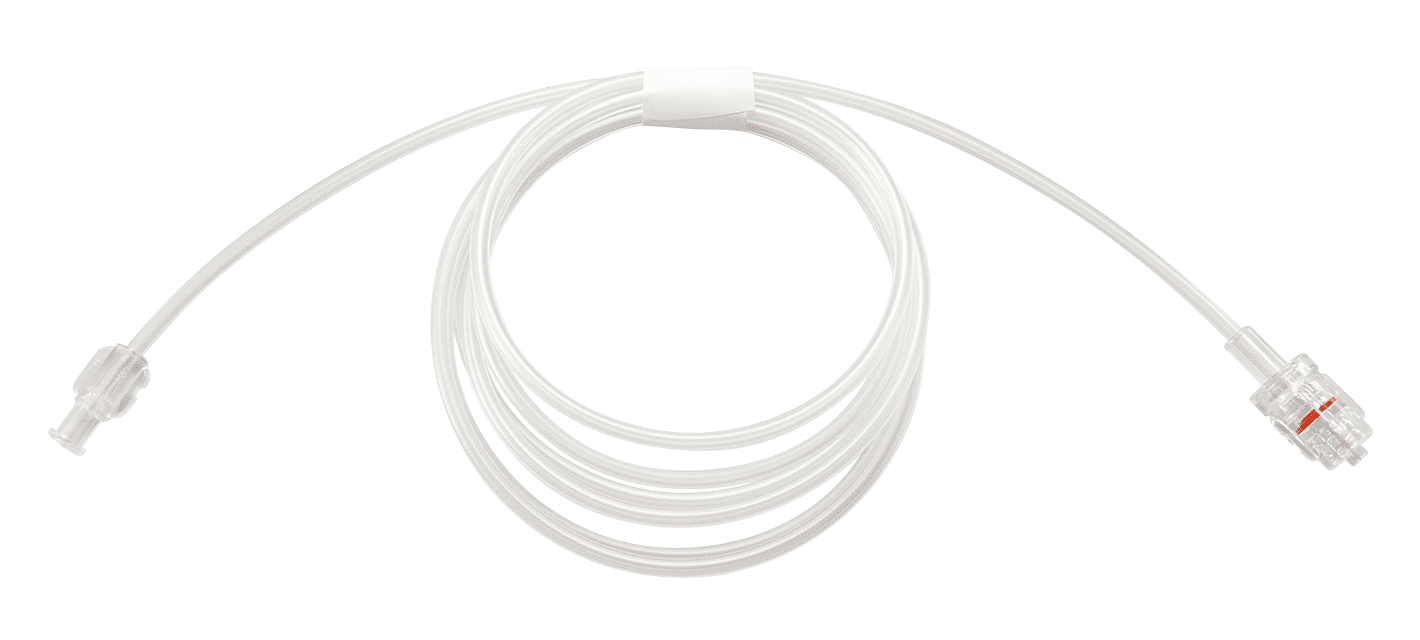
FLUID ADMINISTRATION TUBING IN CRITICAL CARE
Adequate fluid administration is necessary for patients to receive the medications and fluids they need while in the ICU. Merit Medical’s fluid management products are designed to effectively manage fluid levels of critically ill patients. Merit Medical tubing, in particular, is available in longer lengths, which can help reduce the risk of exposure between patient and nursing staff as well as conserve personal protective equipment (PPE).
“To reduce the risk of exposure, hospitals are adding extension tubing to the pump’s primary administration set and then running it under the door into the hallway. This helps to decrease the amount of exposure a frontline healthcare worker has when repeatedly entering a patient’s room to manage infusions,” Olsen explains. “Merit’s fluid administration systems are available with tubing up to 108 inches, or 274 centimeters, which have helped decrease proximity of patients and staff.”
MERIT MEDICAL PRODUCT PICK
Merit Fluid Administration Tubing
Key Features & Benefits:
- Available in longer lengths
- Offered in three material configurations (highly flexible polyurethane to clear PVC)
- Provides both a fixed male connector and rotating adapter connector
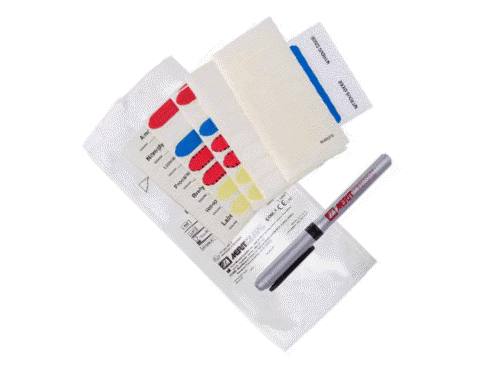
LABELING SYSTEMS IN THE ICU
Patients in the ICU with COVID-19 or flu may require extensive tubing, such as IV and auxiliary lines, to help them recover. Keeping critical care lines accurately labeled is crucial for patient safety.
“Having tools in place that organize patient lines can help clinicians easily identify which fluid line is responsible for what,” Olsen says. “Merit offers Critical Care Label Sets, a labeling system created in response to a customer’s request to help manage multiple IV lines when caring for patients with COVID-19.”
Color-coded with large, legible print, Merit’s Critical Care Label Sets (CCLS) provide a convenient and safe identification system for IV lines, which can help determine each line’s purpose accurately and efficiently, reducing the potential for medication errors.
MERIT MEDICAL PRODUCT PICK
CCLS
Key Features & Benefits:
- Streamlines medication labeling
- Designed to stick when wet
- Comes with a sterile, fine-point PAL™ pen that is smear resistant
INFECTION PREVENTION SYSTEMS IN CRITICAL CARE
According to the CDC, on any given day, about one in 31 patients has at least one healthcare-associated infection (HAI).
“With the number of COVID-19 cases in the ICU rising, infection prevention—and protecting all lines—is critical now more than ever,” Olsen explains.
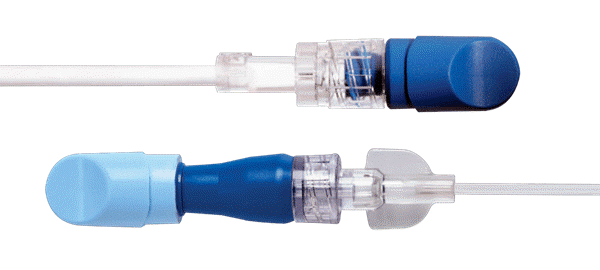
To provide effective infection prevention, Merit Medical offers the DualCap® Disinfection & Protection System, a uniquely designed disinfecting cap system for use on both the male luer connector at the end of the IV tubing and the needle-free valve.
Invented by two infusion nurses, the DualCap System helps to prevent intraluminal contamination as well as device cross-contamination, which is especially important for IV catheter use over extended dwell times.1 The DualCap System can be used on all lines—both central and peripheral—disinfecting the male luer connector and the needle-free valve in 30 seconds, providing a physical barrier to contamination for up to 7 days if not removed.2
MERIT MEDICAL PRODUCT PICK
DualCap Disinfection & Protection System
Key Features & Benefits:
- The DualCap System not only provides built-in disinfection, but protection that comes from a secure connection
- Both caps contain medical-grade sponges saturated with 70% isopropyl alcohol (IPA)
- Both caps feature an easy-to-grasp, hold and twist tapered design that fits tightly and securely
Learn More
You can also explore virtual training tools that can help you in-service your teams with step-by-step instructions—all available when you need them.
When treating vulnerable patients, every product you use matters. During this challenging time, nothing is more important to Merit Medical than the health and safety of customers and their patients.
Visit our Critical Care Products and Solutions page to explore the many ways we help clinicians provide treatment that is safe, effective, and efficient.
REFERENCES
- Mermel LA, “What Is the Predominant Source of Intravascular Catheter Infections?” Clin Infect Dis 52, no. 2 (2011): 211 ̶ 212. PMID: 21288845.
- DualCap IV Pole Strips® Instructions for Use, 2016. Catheter Connections, Inc.
- Avomeen Analytic Services. Testing Report Prepared for Catheter Connections. October 2013.
“When You Are Informed, You Hold the Power” – Sharon Huff, SCOUT® Patient

“A close friend and I are going through this breast cancer journey together. She is a few weeks ahead of me in her diagnosis, so I have learned a lot from her experience,” Sharon said. “She had a wire localization and told me, in her words, it was going to freak me out. She had the wire put in very early the morning of her surgery and had to be still, so the wire wouldn’t move. Moving the wire could result in not being able to accurately locate the cancer.”
It came time for Sharon’s surgical consult with Dr. Jonsson at Kaiser Ontario Medical Center, and she was expecting to learn of her surgery date and hear about the wire localization procedure. This weighed heavily on Sharon’s mind. When Dr. Jonsson told her she would be having a wire-free localization, she was pleasantly surprised and relieved.
Although Sharon has an extensive educational background and is an avid reader and researcher, she had never heard of a reflector placement with SCOUT®. She immediately turned to the internet. Sharon read everything she could find about the procedure and watched videos to familiarize herself with what the reflector looked like and how it was placed.
When it was time for her reflector placement with Dr. Nguyen of the radiology department at Kaiser Fontana Medical Center, she was well informed and prepared. “The procedure to implant the reflector was easy and did not take long at all,” Sharon commented. “I was very impressed.”
COVID-19 adds additional stress to any medical procedure. In Sharon’s case, her husband was not allowed to accompany her and instead had to wait in the car. On the day of surgery, she only had to arrive two hours prior to the surgery start time, making her husband’s wait time much shorter than if she would have needed a wire localization on the day of surgery. “Having that little reflector made our day so much smoother and shorter,” Sharon recounted.

When asked to share pearls of wisdom for individuals going through the breast cancer journey, Sharon had three: inform yourself, make your own decisions, and advocate for yourself. “When you are informed, you hold the power,” Sharon said. “And you can feel better about your choices.”
Disclaimer: This information is not intended nor recommended as a substitute for medical advice, diagnosis or treatment. Always seek the advice of a qualified physician regarding any medical questions or conditions.
Merit Streamlines basixCOMPAK™ Kits, Provides Options to Better Meet Clinician Needs

To enhance our inflation device offerings, starting this month we’ve streamlined kits for the basixCOMPAK™ Inflation Device. These inflation device kit options are designed to deliver better product optimization, increase inventory availability, provide efficient product deliveries, and create added pricing transparency.
At Merit Medical, we understand what makes an exceptional inflation device, and we’re proud to innovate and improve our products, delivering the solutions you need for your interventional procedures. This commitment to excellence allows us to provide you with a portfolio that remains inventive yet reliable with kits that better meet your needs, offering the security and confidence in performance you expect from every Merit product.
Browse our Inflation Device portfolio and contact our Customer Support team to see how our product solutions can fit the requirements of your lab.
Give Yourself a Pay Raise in 2021—Work at Merit
The New Year is a prime time for job seekers. Despite the pandemic, “changing careers” is still a major job prediction and trend expected for 2021. When looking for a new gig, it’s no surprise that the number-one factor employees and job seekers look for is salary.

“Merit continually evaluates its position in the market,” says Rick Portrey, Director of Recruiting at Merit. “We want to adjust to take care of our employees—from entry level on up.” According to Portrey, this pay increase will enable employees to stress less about their paycheck and focus on their path at Merit, building a long-term career at the company.
In addition to helping meet long-term professional goals, Merit’s pay increase will also provide more opportunities to advance employee financial objectives. Whether this means paying off debt, saving for retirement, purchasing a home, or just feeling more financially secure—Merit’s vision for the pay increase is to help employees experience financial security.
“Merit supports its employees, and an increase in pay is one way we’re showing that,” Portrey went on to explain. “We invest in our employees by valuing their time and productivity,”
A 2020 Recruiter Nation Survey showed that top priorities since the onset of the pandemic have also included mental health benefits, shift flexibility for working parents, and COVID-19 safety protocols.
“Merit prides itself on meeting a number of employee needs, including shift flexibility, a commitment to health and wellness, and keeping employees safe,” Portrey says. “Combined with the new 2021 pay increase, Merit is set to emerge as a premier employer for anyone looking for a career boost.”
Learn about the precautions Merit is taking to keep employees safe during the pandemic and watch Merit’s Journey Through COVID-19: A Video Series.

If you’re searching for a workplace that will invest in your success, browse our job openings here. After filling out an application, you can expect a response within two business days.
For more reasons on why Merit is a great place for those changing careers, visit us here. To explore tips on how to apply at Merit—and land the job—visit us here.
We look forward to working with you!